Abstract
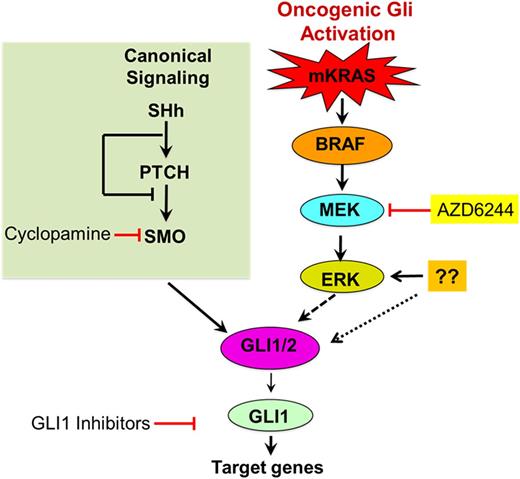
Hematopoietic stem and progenitor cell (HSPCs) proliferation are tightly regulated during normal homeostasis. Recent data suggests that aberrantly activated signaling pathways such as Hedgehog (Hh), Notch, and Wnt occur during early hematopoietic oncogenesis. Gli genes (Gli1-3), known as Gli-code, encode Cys2-His2 zinc finger transcription factors governing the expression of pro-survival genes at the distal end of the Hh pathway (Hh→PTCH1→SMO→GLI). These genes are tightly regulated in embryonic development. They are a common node of dysregulated activation through which many oncogenic signals, such as Hh, FLT3, PI3K-AKT, TGFβ, and RAS, converge. Hh-signaling critical for embryogenesis is mostly inactive in adult tissues; however, aberrant activation of the Hh/Gli has been implicated in cancer progression and chemotherapy resistance in AML. Activation of SMO triggers an intracellular signaling cascade leading to the formation of active Gli transcription factors which functionally converge on Gli1. In addition to canonical PTCH/SMO-dependent regulation of Gli1, we have shown that Gli-signaling axis can be activated independent of Hh-signaling (Fig.1).
The specificity of the Gli pathway in leukemic cells suggests that Gli inhibition may have clinical utility. Most efforts have been devoted towards therapeutic targeting upstream of the Gli Hh-signaling through PTCH/SMO. These efforts remained mostly ineffective due to convergence of parallel oncogenic signals at Gli1 irrespective of Hh-signaling status.
We have focused our structure-guided small molecule design efforts to develop potent Gli1-inhibitors, a nodal point of the convergence of various oncogenic signals (Fig.1). Our Gli1 inhibitor prevents both Gli1 binding to DNA and transcription of Gli-target genes, inducing cell death specifically in leukemic cells while sparing normal CD34+ hematopoietic cells. Our inhibitor binds on the Gli1-DNA interface between Zn-finger 2 and 3 (KD= 14nM) and is >200-fold more effective than the previously reported Gant61 Gli1 (KD=3.2µM) at inducing cell death of leukemic cells in semi-solid methylcellulose-based media. We performed a colony forming assay on CD34+ bone marrow cells derived from RAS mutant patients in the presence and abscence of our highly potent iGli1 inhibitor. iGLi1 demonstarted high potency (IC50, 50nM) in preventing colony formation in a dose dependent manner while no cytotoxic effect was observed on normal bone marrow cells. The LD50 for normal bone marrow cells was 10µM providing a therapeutic index of more than 200. Cellular mRNA sequencing analysis was also performed in MOLM13 and MV4-11 cells to confirm the downregulation of Gli1 target genes. Consistent with the Gli1 inhibition, iGli1 treatment of these AML cells led to the downregulation of Gli1 target genes Gli1, Gli2, PTCH1, Cyclin1/2, P21, P27, and IKK-β. iGli1 inhibited the binding of Gli to DNA and rapidly inhibits transcription of critical Gli-dependent target genes. Furthermore, inhibition of Gli1 resulted in the stalling of RNA Pol II, leading to redistribution of pause and pause-release factors DSIF/NELF and PTEFb, respectively. We also observed that iGli1 treatment of MOLM13 or THP1 cells arrests cell cycle progression in S-phase. Inhibition of Gli-dependent transcription by iGli1 leads to accumulation of R-loops (RNA:DNA hybrids) that cause a robust DNA damage, inhibition of DNA replication, S-phase progression, and subsequent apoptosis.
The efficacy of iGli1 was also compared to standard care used in the treatment of AML (Cytarabine and Idarubicin under in vitro conditions against MOLM-13, THP-1, K562, and MV4-11). We observed that iGli1 was superior to cytarabine/idarubicin treatment in cell culture model system as a single agent. In addition, ex vivo exposure of MV4-11 cells with iGli1 completely shut down the tumor formation in NSG mice in a xenograft model.
In summary, we have shown that Gli1 is not only a strong target to treat AML patients but also an excellent approach for developing a novel therapeutic for patients where Gli1 expression and activity is high, such as patients with RAS mutations. The Gli1 inhibitior is highly effctive as a single agent against highly aggressive form of AML with an excellent therapeutic index.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal